ZrO2 Zirconia ceramics
What kind of material is zirconia ceramic
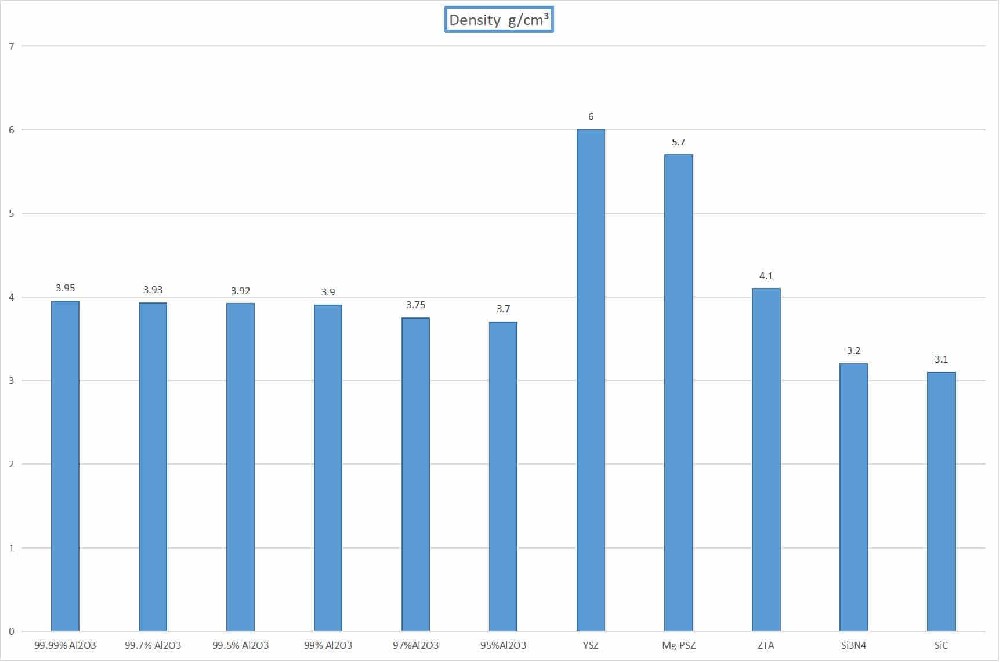
1, Zirconia ceramics or zirconia ceramics are very strong technical ceramic materials, with excellent hardness, toughness and corrosion resistance, no other ceramic materials common brittleness. There are several grades of zirconia ceramics, of which yttrium oxide and magnesium oxide partially stable zirconia ceramics are the most common.
2, Zirconia toughened alumina (ZTA) ceramic is a composite material with alumina and zirconia as the main elements. The structure of ZTA consists of zirconia grains placed in an alumina matrix. Compared to alumina, it has additional strength and toughness and costs less than zirconia.
3, The various types of zirconia ceramics are developed from the desire of manufacturers to enhance the performance of different phases of zirconia ceramics. Each stage requires processing suitable for use in a variety of environments.
4, Zirconia toughened alumina is one of many types of phase.
5, The process of manufacturing zirconia ceramics involves the calcination of zirconium, which involves heating the material to extreme temperatures, but limiting the oxygen content. The initial step of the process is to remove any impurities and produce thermal decomposition
Basic characteristics
Zirconia ceramics are advanced ceramic materials with zirconium dioxide (ZrO₂) as the main crystal phase. Their core characteristics include:
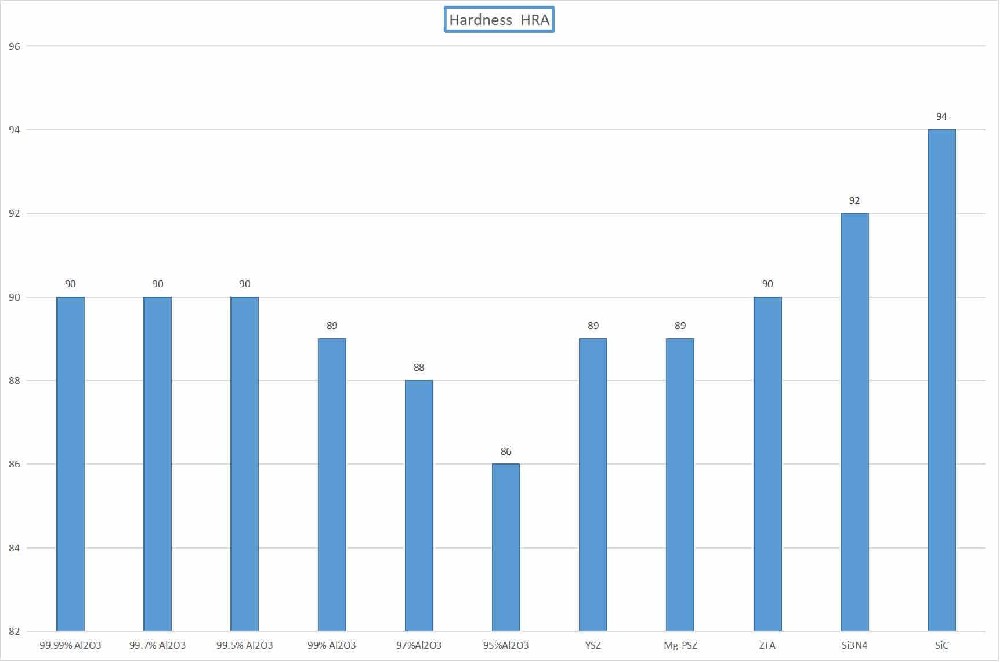
High hardness and wear resistance
The Mohs hardness can reach over 7.5, and some ceramics have a hardness of over 9, second only to diamond.
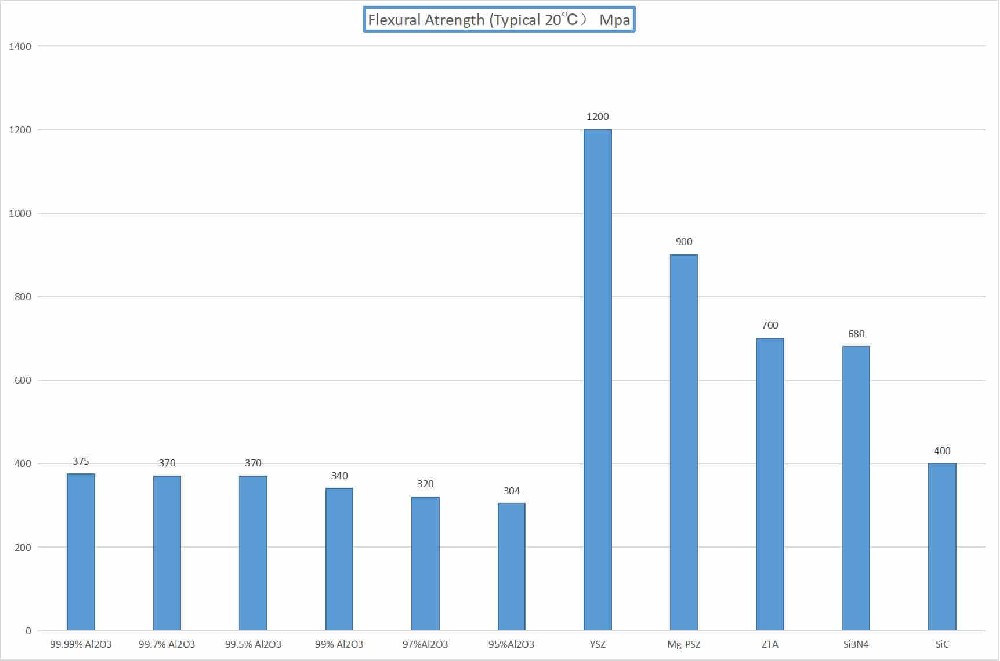
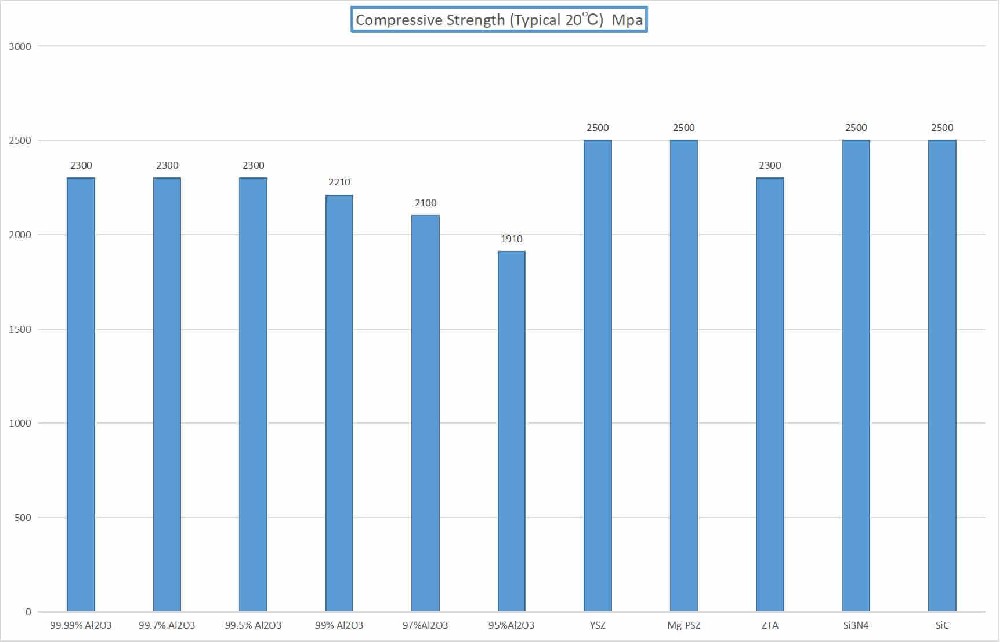
High strength and toughness
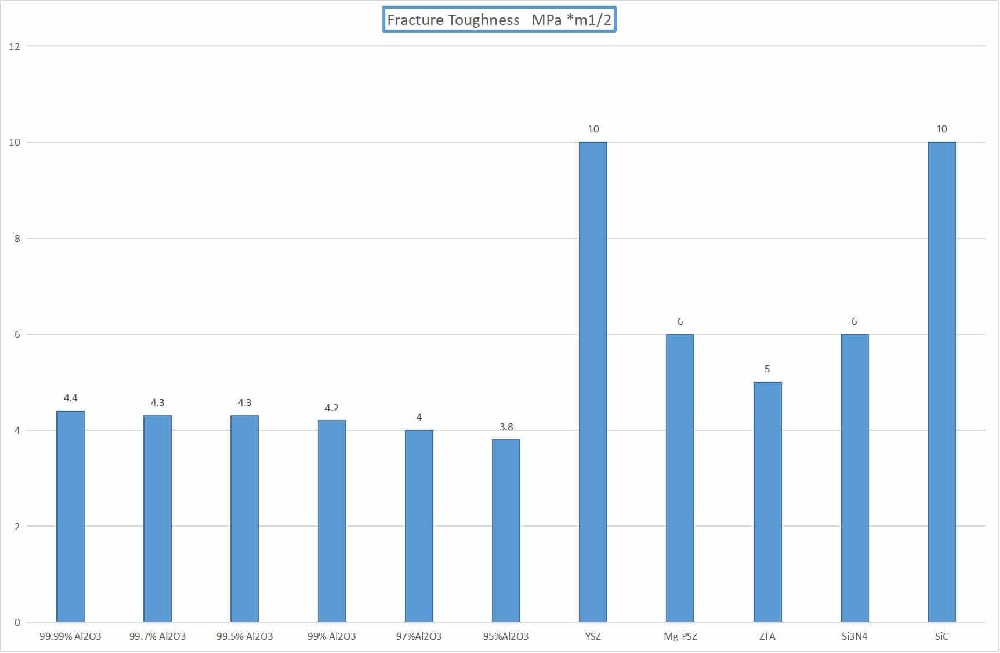
Fracture toughness up to 12MPa·m^(1/2), flexural strength up to 1100MPa, significantly better than traditional ceramics.
Heat resistance
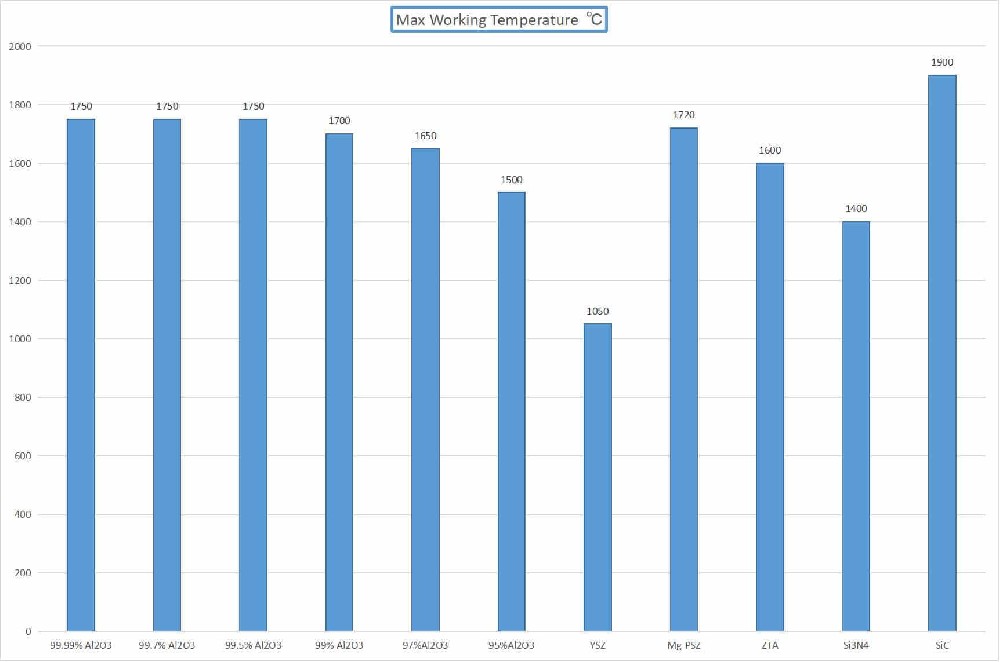
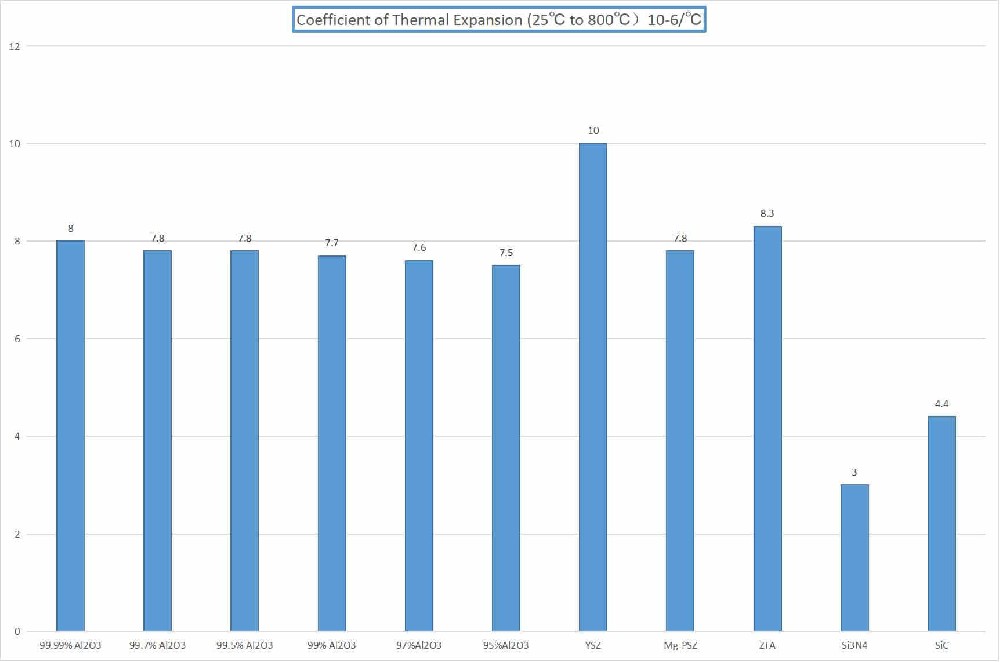
Melting point about 2715 ° C, coefficient of thermal expansion similar to that of steel, suitable for use in high-temperature environments.
Chemical stability
Shows inertness to acids, bases and biological tissues, and is commonly used in dental implants and biomedical fields.
Physical and chemical properties
Appearance
Pure zirconia is white, yellow or gray in the presence of impurities.
Crystal structure
There are monoclinic phase (mZrO₂), tetragonal phase (tZrO₂) and cubic phase (cZrO₂), and phase transformation can enhance the toughness of the material.
Electrical properties
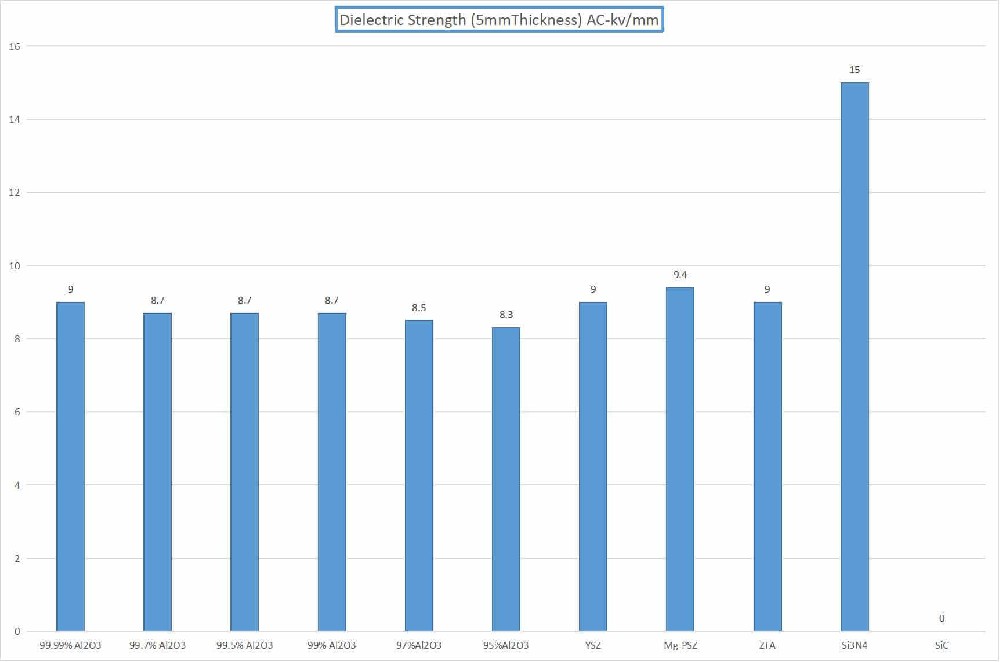
Insulating at room temperature, conductive with oxygen ions at high temperatures
Zirconia (Zirconia), with the chemical formula ZrO₂, is an important ceramic material with excellent physical and chemical properties. It is an oxide of zirconium and is widely used in multiple fields, such as ceramics, electronics, metallurgy, catalysts, etc. This article will conduct a detailed discussion on the properties, preparation methods, application fields and future development trends of zirconia.
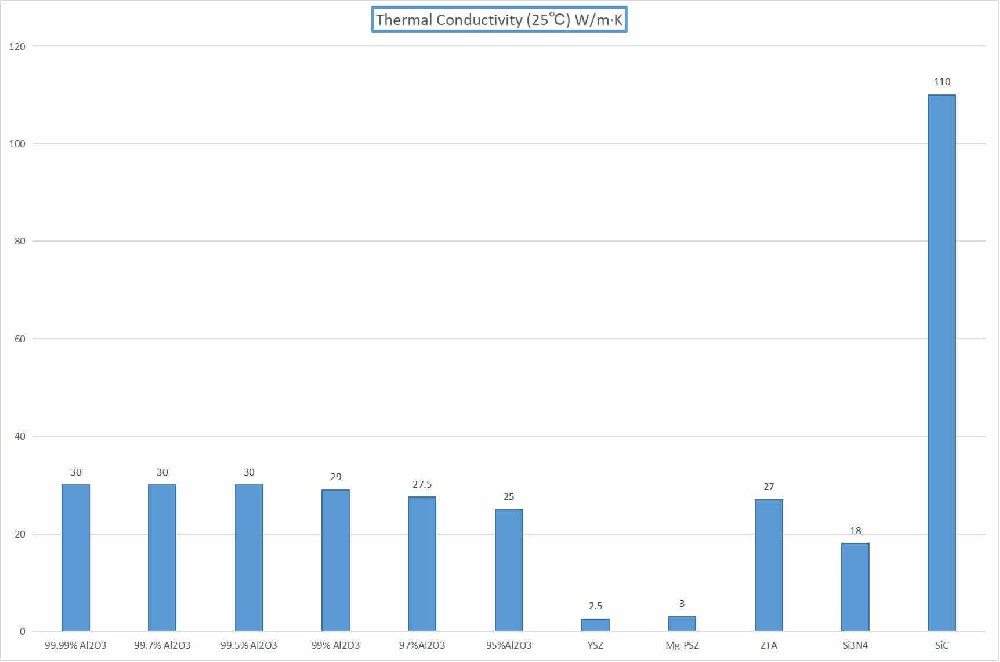
The physical and chemical properties of zirconia make it stand out among many materials. Zirconia has excellent high-temperature resistance, with a melting point as high as 2700 degrees Celsius, which enables it to maintain good stability even in high-temperature environments. Zirconia has a relatively low coefficient of thermal expansion. Compared with other ceramic materials, it has stronger thermal stability and can resist the stress caused by temperature changes. Zirconia also has excellent corrosion resistance and wear resistance, which enables it to maintain good performance even in some harsh environments.
The structural characteristics of zirconia are also very unique. There are three main forms of its crystal structure: monoclinic, tetragonal and cubic. At room temperature, zirconia mainly exists in the monoclinic phase, while at high temperatures, it transforms into the cubic phase. The characteristics of this phase transition make zirconia have excellent toughness and strength in high-temperature environments, and it is often used to manufacture high-temperature ceramics.
There are various methods for preparing zirconia. Common ones include solid-phase synthesis, sol-gel method, and chemical vapor deposition method, etc. Among them, the solid-phase synthesis method is the most traditional and commonly used one. It involves mixing zirconium compounds with oxides and heating them to cause a chemical reaction and generate zirconia. The sol-gel method dissolves the precursors of zirconium in a solvent to form a colloid, and then obtains zirconia ceramics through drying and sintering. Both of these two methods have their own advantages and disadvantages. When making a specific choice, a comprehensive consideration should be made based on the application requirements.
Phase transformation and stabilization of zirconia
The transformation between the tetragonal phase and the monoclinic phase is a martensitic transformation, whose characteristics include: Firstly, this process has the characteristics of nucleation and growth, and no diffusion occurs during the phase transition, which takes place within a wide temperature range; Secondly, atoms undergo ordered displacements, but the displacement amount is less than the distance between one atom, and adjacent atoms remain adjacent after the phase transition. Furthermore, this phase transition is accompanied by a significant volume change (3%-5%) and shear strain (1%-7%), and it is reversible. The opposite phase transition will also occur during heating. The phase transition between the tetragonal phase and the monoclinic phase endows the material with toughness. Treatment with zirconia stabilizer can prevent the fragmentation of pure zirconia. However, pure zirconium dioxide is prone to cause product fragmentation and loss of practical value when undergoing t-m phase transformation. Therefore, in the production process, stabilizers such as Y2O3, CeO2, CaO and MgO are usually used to treat zirconia ceramics. These metal ions such as Y3+, Ca2+, Mg2+, Ce2+, etc. are introduced into the crystal structure to replace Zr4+, thereby forming a solid solution. In this way, tetragonal zirconia can stably exist at room temperature without undergoing phase transformation, thereby stabilizing the zirconia ceramic material.